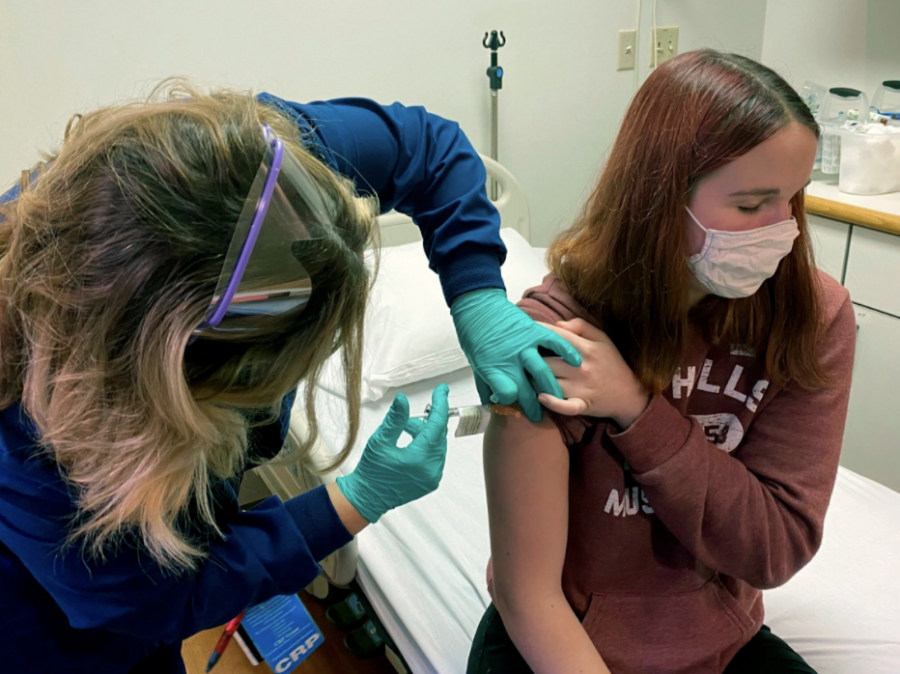
Pfizer Vaccine Now Available for Students Ages 12-15
May 22, 2021
An advisory panel for The United States Centers for Disease Control and Prevention (CDC) authorized the use of the Pfizer and BioNTech COVID-19 vaccine for 12-15 year olds on May 12, after a final vote of 14-0.
The move comes just days after the Food and Drug Administration approved the use of the Pfizer vaccine on an emergency basis on May 10. The final authorization from the CDC expanded eligibility for the vaccine to nearly 17 million more teens in the United States.
“Every person with COVID-19 provides the virus with an opportunity to spread and continue to mutate and further expose our communities,” Dr. Bill Gruber, senior vice president at Pfizer Inc., said. “The decisions from health authorities bring us one step closer to protecting adolescents and achieving herd protection.”
In a trial conducted with over 2,000 teens aged 12-15, Pfizer Inc. found that the vaccine had a 100% success rate in preventing the virus. The side effects of the vaccine in the trial were found to be very similar to those observed in adults, mostly consisting of cold-like symptoms.
“I’m definitely happy now that I’m eligible,” sophomore Satvik Subbaraman said. “I would like to get the vaccine as quickly as possible, because I trust that it is safe and I would like to be safe if I somehow contract COVID-19.”
However, the move does not come without hesitancy. Many officials have criticized the decision to expand vaccine eligibility to an age group that is relatively low-risk for the virus, when many countries such as India are in higher demand for doses. In addition, parents have expressed concern with giving their children the vaccine; according to a study by the Kaiser Family Foundation, just 29% of parents are planning to vaccinate their children immediately, with the remaining 70% either planning to wait or refusing to vaccinate.
“It’s going to be very difficult, if not impossible, for us to reach herd immunity unless our children are also vaccinated,” CNN medical analyst and former Baltimore health commissioner Dr. Leana Wen said.
Nevertheless, the shift has opened pathways for a safer reopening of middle and highschools in the fall. Pfizer has already begun plans to gather data and file a request for vaccinating children aged 2-11 in September.For information on how to get a vaccine, please visit https://www.cityofirvine.org/covid-19-resources/covid-19-vaccine-resources.